Carbon atoms are bonded in different ways or forms, and many homologues such as carbon nanotubes, diamonds, and graphite have been identified. As the possibility of its existence as a carbon allotrope was pointed out, many chemists tried to make it, but the Harvard University and IBM research team succeeded in creating a ring made of only failed carbon atoms.
In carbon nanotubes, one atom is bonded to 3 atoms, but in diamond, carbon atoms can be chemically bonded to other atoms in a variety of configurations, such as the number of atoms bonded to 4 atoms. In addition, since a carbon atom can only bond with two nearby atoms, it has been known that it is possible to create a cyclic carbon allotrope made of only carbon atoms by circulating a combination of carbon atoms on both sides.
These rings of carbon atoms are called cyclocarbons. Numerous chemists have tried to synthesize it, but have failed. The problem was that the chemical reactivity was high compared to the cyclic structure, diamond, and graphene, and the stability when bent was lowered.
The Oxford University research team has continued to work to create these cyclocarbons. The research team first tried a method to remove oxygen atoms in the laboratory by creating a ring containing oxygen atoms as well as carbon atoms and sending the ring to an IBM laboratory in Switzerland.
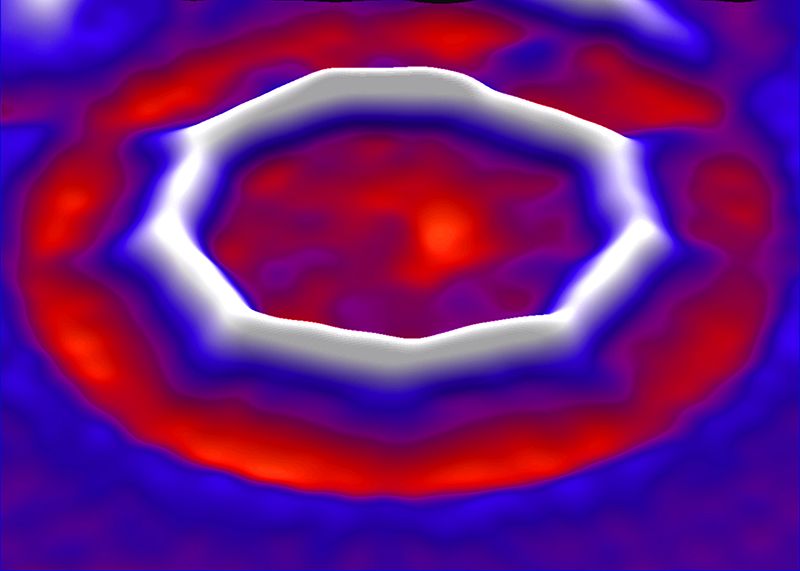
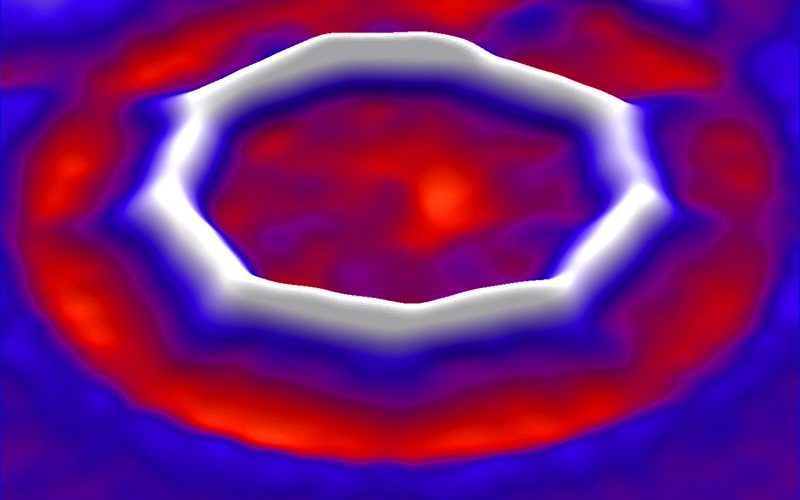
The Swiss research team put a ring sent from Oxford University on a layer of sodium chloride in a vacuum chamber and used electric current to remove oxygen atoms. After trial and error, the research team succeeded in making a carbon-only ring, a cyclocarbon. The completed cyclocarbon has a structure in which 18 carbon atoms are lined up.
When an IBM research team examined cyclocarbons under a microscope, they found that carbon rings have alternating single and triple bonds. Until now, theories about how cyclocarbons are bound have been disagreeable, but they came to a conclusion when they were actually made.
The structure of alternating single bonds and triple bonds gives the properties of cyclocarbon semiconductors, and in the future, it is said that there is a possibility to apply this property to molecular-sized electronic components. The research team continues to investigate the properties of cyclocarbons, and plans to conduct research on the mass production method of cyclocarbons. Related information can be found here .

















Add comment