It is reported that Moderna Therapeutics has completed initial development of a vaccine against the novel coronavirus, COVID-19, and has completed a proposal to conduct clinical trials for the National Institute of Allergy and Infectious Diseases NIAID. . The test starts in April.
The developed mRNA vaccine is said to have been completed only 42 days after the Corona 19 genome analysis was conducted in China this year and the results were provided. It takes time to make a vaccine while cultivating a large amount of virus if it is like before, but it has been successfully developed early by using new genetic engineering.
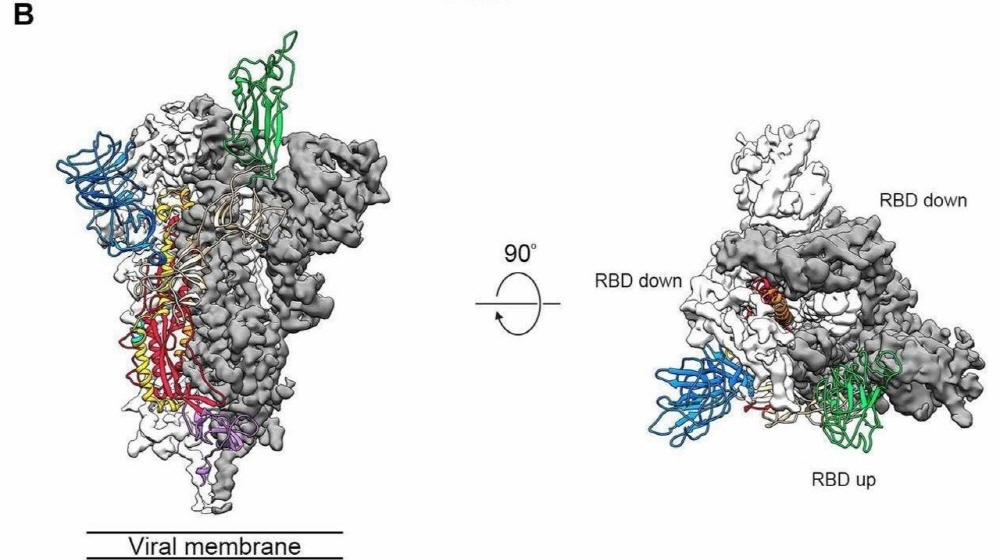
If the mRNA vaccine is finished so that immunity using the Corona 19 protein can be obtained in the body, the faster it is completed, the more people can be protected from infection.
In addition, clinical trials are in progress in the US to see if remdesivir can be used as a treatment for patients who tested positive. Remdesivir has been effective when administered to SARS and MERS patients. Even if there is no immediate effect on patients infected with Corona 19, it is believed that the effect of suppressing the spread of infection will be great. Atomic-level clarifications are underway in countries around the world, and as effective countermeasures and treatments are being sought, attention is focused on whether such efforts will lead to an end to Corona 19. Related information can be found here .

















Add comment