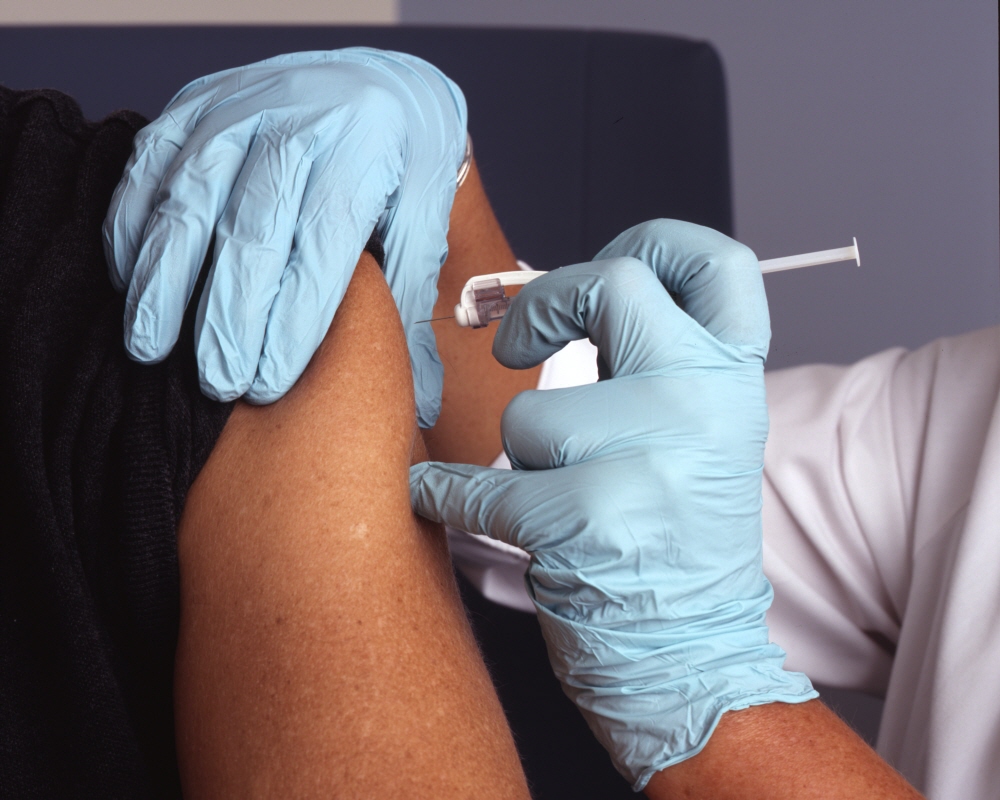
The COVID-19 vaccine BNT162b2, developed by Pfizer and Biontech, has been rapidly inoculated since the FDA approval of the US Food and Drug Administration in December 2020. How was Pfizer and Biontech’s COVID-19 vaccine made?
This vaccine is an mRNA vaccine that uses mRNA transcribed from DNA that produces the artificially cloned COVID-19 spike protein to trigger an immune response. At the Pfizer facility in Chesterfield, Missouri, a circular DNA called a plasmid containing DNA for the spike protein is stored. E. coli introduced with this plasmid is cultured for 4 days to produce trillions of copies of the plasmid.
After 4 days of incubation, only the plasmid is taken out by adding a treatment to destroy E. coli. Afterwards, it is checked whether the DNA base sequence of the spike protein contained in the extracted plasmid has not changed since it was copied by E. coli.
Next, the plasmid is cut with a restriction enzyme, the circular plasmid linear spike protein DNA is extracted, filtered, and then filled into a 1 liter bottle. After reconfirming the DNA sequence in the bottle, it is sent to the Pfizer facility in Andover and the Bio&Tech facility in Mainz, Germany, while maintaining the temperature at minus 20 degrees Celsius. In addition, it is said that it can produce 1.5 million doses of COVID-19 vaccine per 1 liter bottle.
At the Andover facility, five bottles containing DNA are extracted over the course of a day, mixed with enzymes and mRNA components necessary for transcription into mRNA, and transcription is performed for several hours. After transmission, the mRNA is sent to a Michigan facility at minus 20 degrees Celsius for processing into vaccines after removing unnecessary DNA enzyme impurities. Samples are also sent to the Chesterfield facility for sequencing.
At the Kalima state facility, mRNA is extracted, mixed with water, and mixed with lipids to make it easier to enter human cells to create lipid nanoparticles. It is said that the generation of these lipid nanoparticles requires special equipment using microfluidics.
After the completion of the lipid nanoparticles, impurities are removed and disinfected, and then the vaccine is sealed in a glass bottle. The Kalamazoo facility is said to contain up to 575 vials per minute of the vaccine.
When the bottle is sealed, the vaccine is cooled down to minus 70 degrees Celsius and quality tests are performed at facilities in Chesterfield and Andover for about four weeks. Pfizer is said to be carrying out a 60-day process from DNA generation to completion of the vaccine, and more than half of this period is spent on quality testing.
Along with the spread of COVID-19 infection, the existence of variants that do not work well with existing vaccines are also being reported. According to reports, Pfizer and Biontech are developing a new version of a vaccine against the mutant, and they will soon be able to mass-produce a vaccine that is effective against a specific mutant. Related information can be found here.

















Add comment