
Snoring is a headache not only for you, but also for the person sleeping next to you. The problem is that in addition to being noisy and unable to fall asleep, people who snoring may have obstructive sleep apnea.
However, there are still not many alternatives to treat snoring or sleep apnea. Sleep apnea treatment is sometimes done with CPAP or positive pressure, but not all of them are worn while sleeping. While some people can use it well to sleep deeply, the device may be annoying. However, eXciteOSA, newly approved by the US Food and Drug Administration, is a new concept of treatment that is used only for a short time while awake, not when sleeping.
Obstructive sleep apnea causes the tongue and neck muscles to loosen while sleeping, causing the airways to close. Excite OSA stimulates the muscles of the tongue while awake and trains in a difficult state of sleep. It’s like a tongue muscle training that stimulates the tongue for 20 minutes by biting it in your mouth.
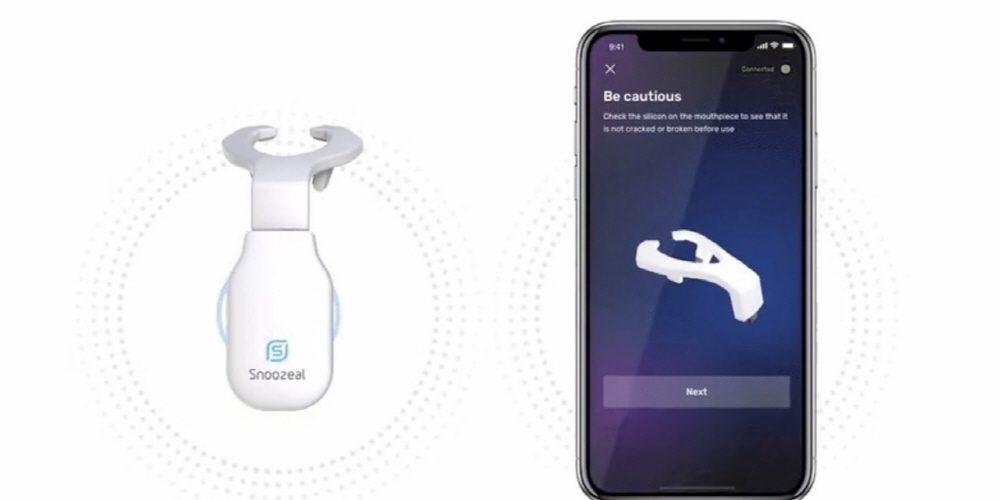
Excite OSA is a mouthpiece device that is used by mouth and has 4 electrodes, 2 on the top and bottom of the tongue. A weak current that stimulates the muscles flows from the electrode, and the current intensity can be adjusted with a smartphone. The treatment is first 6 weeks, using the device for 20 minutes a day, but then once a week.
According to an FDA-verified experiment, Excite OSA reduced snoring larger than 40dB by 20% in 87 out of 155 patients participating in the experiment. In addition, for those who suffered from mild sleep apnea as well as the nose, the apnea hypopnea index AHI, which indicates the severity of symptoms, fell by an average of 48% in 41 out of 48. However, in the FDA review of the side effects, it is reported that there were increased saliva, tongue and tooth pain, tongue numbness, irritable metal taste sensation, nausea, and jaw strain.
Excite OSA requires a prescription and cannot be used by people wearing pacemakers, temporary or permanent implants, orthodontic appliances, or jewelry worn on teeth. In addition, a thorough dental examination is required before starting treatment with Excite OSA. This product is intended only for minor sleep apnea, except for those with severe symptoms.
An increasing number of people suffer from obstructive sleep apnea and can lead to serious symptoms such as glaucoma, diabetes, heart disease, cancer, and cognitive behavioral disorders without treatment. However, it is a common disease, and a study by the U.S. Department of Health and Welfare estimates that 936 million of adults aged 30 to 69 around the world experience more than mild obstructive sleep apnea. A Johns Hopkins University study found that 45% of adults snoring occasionally and 25% all the time. Snoring in itself is not necessarily equated with a serious sleep disorder, but most of the symptoms overlap with obstructive sleep apnea.
Obstructive sleep apnea not only affects sleep quality, but can lead to other serious disorders if left unattended. The approval could offer new alternatives to people who have experienced nasal and mild sleep apnea. Although Excite OSA may not be a special drug, it is producing promising results, and there is a possibility that there will be more treatments inspired by this product. Related information can be found here .

















Add comment